The Earth’s atmosphere has strengthened its ability to remove air pollutants, including the potent climate-warming gas methane, according to research published in Nature Communications.
Regarded as a breakthrough for climate science and the understanding of atmospheric chemistry, the study of the atmosphere’s self-cleansing ability focused on determining the quantity of its elusive driver, the hydroxyl radical (OH), dubbed the “detergent of the atmosphere” by Nobel Prize winner Paul Crutzen.
By applying an advanced method to analyze two long-running measurements of air samples from New Zealand and Antarctica dating back to the late 1980s, the research by New Zealand’s National Institute of Water and Atmospheric Research (NIWA) revealed a significant trend in the atmosphere’s self-cleansing capability.
The research highlights that without the increased cleaning capacity of hydroxyl, methane would have contributed even more to global warming.
The long-term study by NIWA scientists, together with researchers from Victoria University of Wellington, GNS Science, and a collaborator from Finland, reveals the atmosphere’s self-cleansing ability has been strengthening in the Southern Hemisphere since about 1997.
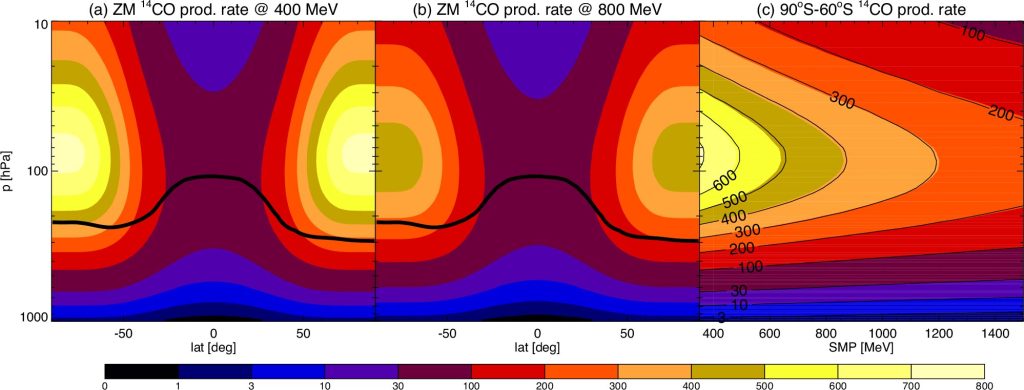
The 33-year scientific investigation concentrated on the atmosphere’s strongest oxidant, OH, and identified radiocarbon monoxide (14CO) as a reliable tracer. The ultra-rare form of carbon monoxide is produced when cosmic rays hit the Earth’s atmosphere, with its production rate well understood, along with its removal by OH.
OH is highly reactive and very short-lived, says NIWA Atmospheric Scientist Sylvia Nichol. “OH is a tiny chemical scavenger. Made up of one hydrogen and one oxygen atom, with a free unpaired electron, it is formed in the atmosphere when ultraviolet light from the sun strikes ozone in the presence of water vapor.
“It reacts with harmful trace gases including carbon monoxide and methane in the lowest layer of the atmosphere, the troposphere, which extends up to an average height of 11km (36,000 feet) from the earth’s surface.
“It was a major discovery in the 1970s that OH is produced in the troposphere by reactions to allow the oxidation of gases such as carbon monoxide, methane, and ethane. Even though OH’s lifetime may only be less than a second or so, it plays a vital role in cleansing the atmosphere.”
With the highly reactive hydroxyl controlling the atmospheric lifetime of most gases, the presence of OH is critical for controlling concentrations of some greenhouse gases, particularly methane, says Nichol. “Even though the hydroxyl radicals appear in tiny quantities for a short time, they remove carbon monoxide and nearly 90% of methane in the air, so it is vital for maintaining air quality.”
The dynamic nature of OH, along with its very low concentrations, means it is notoriously difficult to observe and accurately quantify directly, says NIWA Principal Technician Gordon Brailsford, who has spent decades collecting air samples.
Discover the latest in science, tech, and space with over 100,000 subscribers who rely on Phys.org for daily insights.
Sign up for our free newsletter and get updates on breakthroughs,
innovations, and research that matter—daily or weekly.
“Ultraviolet light influences hydroxyl production, so levels of this atmospheric cleaner have very large fluctuations on a daily and annual basis. OH is only formed during daylight hours, meaning it drops down to almost zero at nighttime, and is more prevalent in summer.”
Past attempts to monitor trends in OH have used methyl chloroform, but that has been phased out under the 1987 Montreal Protocol to protect the ozone layer, making it impractical to use, says Brailsford.
“Traditional methods and models predicting hydroxyl’s abundance based on methyl chloroform and other similar industrial gases also produced conflicting inferred estimates of changes in hydroxyl levels and its capacity to cleanse the atmosphere. So instead, we used naturally produced radiocarbon monoxide (14CO), a tracer whose production by cosmic rays we understand much better, enabling us to work out a trend in its removal rate by OH over a long period of time.”
Records from two remote Southern Hemisphere monitoring stations dating back to the late 1980s have yielded quality data for analysis, says Brailsford. “Regular and consistent measurements spanning 33 years at two sites provide the first evidence for a long-term OH increase.
“The Baring Head Atmospheric Research Station outside New Zealand’s windy capital, Wellington, is internationally recognized for its long-term monitoring of clean air.
“Some 4,000km (2,500 mi) further south, the joint New Zealand—U.S. Arrival Heights laboratory on Antarctica’s Ross Island is far away from human contamination, with air samples being collected even during the five months each year of darkness. Both measurement series are by far the longest and most consistent records in the world for 14CO as a tracer for changes in atmospheric chemistry.”
Processing the samples requires many steps, says Principal Technician Rowena Moss, who has devoted more than 10,000 hours to the project. “Large samples of air up to 1,000 liters, were collected in gas cylinders, then dried, compressed, cooled to remove ambient CO2, and concentrated down to a microscopic amount of carbon monoxide and its isotopes.
“These procedures are undertaken so samples can be sent for 14CO measurement by accelerator mass spectrometry at GNS Science’s radiocarbon-dating laboratory. Quality control is essential throughout these different steps to determine the original air sample 14CO concentration.”
The samples from the two different observation stations have proven insightful into the role of OH, says lead author of the journal paper, atmospheric and climate scientist Dr. Olaf Morgenstern, whose work has extended an earlier developed “chemistry-climate” model.
“New Zealand data since 1997 shows a 12% (± 2%) annual decrease in 14CO. Measurements from Antarctica show an even larger 43% (± 24%) drop but only during the December-January period, the height of the Southern Hemisphere summer.
“These research findings suggest that the atmosphere’s oxidizing capacity, driven by hydroxyl, has been strengthening over recent decades. The findings confirm and support our models and corroborate with those from around the world which suggest OH has been increasing globally.”
The researchers examined which processes and atmospheric compounds bring about changes in OH levels, identifying three main drivers of hydroxyl increase, and one driver dampening the increase of OH. “Increasing hydroxyl trends are driven by nitrogen oxides primarily produced by motor vehicles, industrial combustion, lightning and wildfires.
“Hydroxyl is also affected by stratospheric ozone depletion, and water vapor, which is increasing under global warming, while OH has a significant offset due to methane, also increasing quickly, which acts to decrease hydroxyl. Knowing these four factors tells us what may lie ahead for OH, particularly that the increase could well turn into a decline due to changes in our activities.”
The increasing trend of OH found in this study implies there have been larger increases in the emission rates of methane than those estimated assuming constant OH, he says. “Or put differently, methane would have contributed to global warming even more had it not been for this strengthening of atmospheric cleaning capacity.
“All four factors—nitrogen oxides, ozone, global warming, and methane—are exhibiting human-induced trends. Human activity is affecting the climate system’s ability to strengthen its oxidizing power. These findings underline the significant role human activities play in shaping the climate system, affecting the capacity of hydroxide to cleanse the atmosphere and maintain air quality.”
More information:
Olaf Morgenstern et al, Radiocarbon monoxide indicates increasing atmospheric oxidizing capacity, Nature Communications (2025). DOI: 10.1038/s41467-024-55603-1
Provided by
National Institute of Water and Atmospheric Research (NIWA)
Citation:
Atmosphere’s self-cleansing ability followed by long-term study (2025, January 31)
retrieved 31 January 2025
from https://phys.org/news/2025-01-atmosphere-cleansing-ability-term.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.