:max_bytes(150000):strip_icc():format(jpeg)/Health-GettyImages-2006410571-767a0afc43794bebad1342c20ca2d645.jpg)
Compounded versions of Ozempic and Wegovy could soon be banned by the Food and Drug Administration (FDA), after the agency announced that the medications are no longer in shortage.
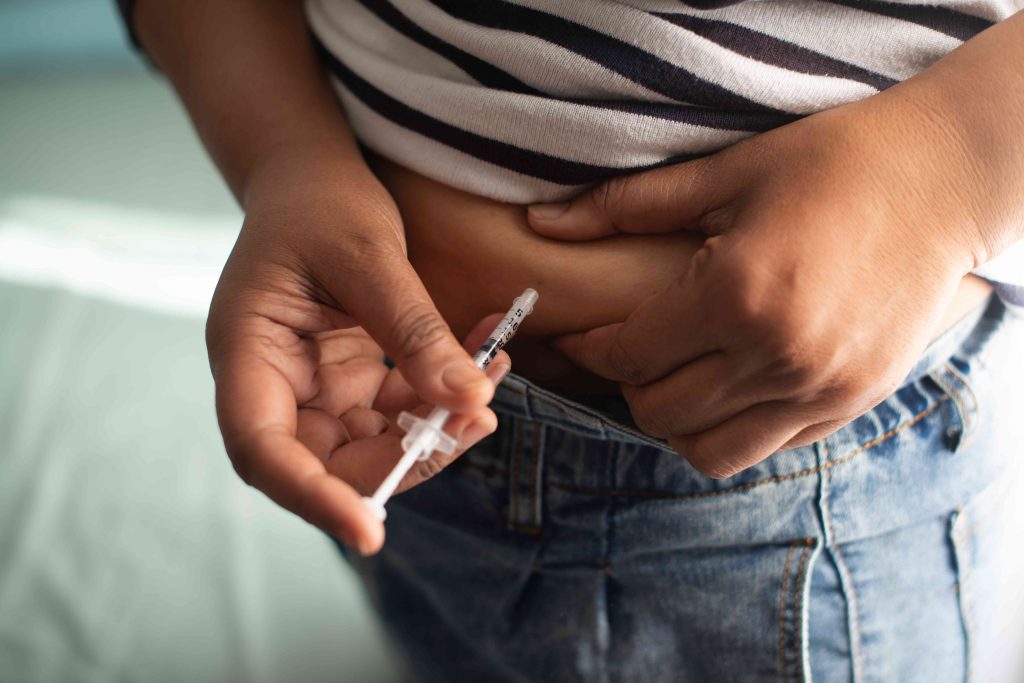
Semaglutide—a glucagon-like peptide 1 (GLP-1) receptor agonist—is the active ingredient in Wegovy and Ozempic. It was added to the FDA’s drug shortage list in August 2022, when demand for the GLP-1 medications skyrocketed in part due to off-label use for weight loss.
To cope with a drug shortage, the FDA allows manufacturers to create compounded versions that use the same active ingredient as the brand-name medication. However, these compounded drugs don’t undergo the same rigorous FDA approval process.
Semaglutide was removed from that list on Feb. 21, once the FDA confirmed with Novo Nordisk, the maker of Ozempic and Wegovy, that there was an adequate supply of injectable semaglutide.
With the shortage of Ozempic and Wegovy over, the agency gave compound drug manufacturers 60 to 90 days to stop making and distributing these medications or face enforcement action, effectively banning compounded semaglutide.
But the Outsourcing Facilities Association (OFA), a trade group representing these compounding pharmacies, has filed a federal lawsuit against the FDA, leaving the fate of compounded semaglutide up in the air for the millions of people who use these products.
Here’s what to know about compounded versions of Ozempic and Wegovy, why the FDA is looking to nix them from the market, and what this all means for safety and access.
Compounded drugs are made by mixing ingredients to create medications that have different formulations than what’s commercially available. They’re usually approved by the FDA when a patient’s needs “cannot be met by an FDA-approved drug,” such as when someone has an allergy to a drug ingredient or there’s a shortage.
Congress does regulate drug compounding, and federal law allows compounding by or under the supervision of licensed pharmacists or physicians in licensed facilities. But these drugs are not FDA-approved, meaning the agency does not verify their safety, effectiveness, or quality before they reach the market.
Despite this, compounded versions of GLP-1 drugs, including Wegovy, Ozempic, Mounjaro, and Zepbound, have become hugely popular in the U.S.
In its lawsuit against the FDA, the OFA said “conservative” estimates show about 2 million American patients were treated with compounded versions of semaglutide from November 2023 to November 2024.
There were about 1.2 million people being treated with Wegovy as of last month, according to the lawsuit.
The years-long semaglutide shortage certainly drove the popularity of compounded weight loss drugs. However, cost is a major factor, too.
Most insurance companies won’t cover semaglutide for non-FDA-approved uses, such as weight loss in those who aren’t clinically obese. This means patients often have to pay out of pocket if they want Ozempic or Wegovy, said Jennifer Carter-Johnson, PhD, JD, an associate professor of law at Michigan State University who specializes in food and drug regulation.
“The branded drug formulations are all patented, meaning that the drug companies can restrict others from selling their forms of the drug and charge higher prices,” Carter-Johnson told Health. “The compounded versions compete with each other on price, and can run about 10% to 15% of the cost of the branded drug.”
That’s led to a booming compounding industry. Large companies such as Weight Watchers, Hers, and Ro—some of which use in-house doctors to write prescriptions—have joined local compounding pharmacies in selling versions of popular GLP-1 drugs, explained Carter-Johnson.
Sun Kim, MD, associate professor of endocrinology at Stanford Medicine, told Health she has many patients who have turned to compounded semaglutide due to its lower cost.
“Most have gotten them from medical spas. Some get them online,” said Kim.
The FDA announced it would delay enforcement of its ban on compounded semaglutide for the time being.
State-licensed pharmacies and physicians have until April 22 to cease production, and outsourcing facilities—which are larger compounders that ship drugs across states without specific prescriptions—can remain in business through May 22. This was to avoid unnecessary disruption in patient treatment.
After the OFA filed its lawsuit, the FDA announced it would hold off on the ban until a district court made a decision or until the previously announced deadlines, whichever comes first.
In its lawsuit, the OFA is arguing that the Ozempic and Wegovy shortage isn’t over and that compounding should still be allowed so that patients have access to reasonably priced medications, explained Carter-Johnson.
“OFA has several legal theories for this argument, but they all center around the allegation that the FDA relied only on data from the branded drug companies rather than examining other data from consumers or the compounding industry,” she said.
Though it’s unclear what the courts will decide, a similar scenario recently took place with tirzepatide, another GLP-1 medication often used off-label for weight loss.
Tirzepatide—an Eli Lilly drug sold under the brand names Zepbound and Mounjaro—had been on the FDA drug shortage list since 2022. The FDA announced that the shortage was over in October 2024, and shortly after, the OFA filed a lawsuit arguing that the drug was still in short supply.
After a judge ruled against the OFA, the FDA issued a new decision in December 2024, giving state-licensed pharmacies until Feb. 18 and outsourcing facilities until March 19 to stop distributing compounded tirzepatide injections.
The FDA appears to be following a similar pattern with compounded semaglutide by delaying enforcing a compounding ban until the court decision, said Carter-Johnson.
If a court decision is rendered against the OFA, the ban’s “timeline is likely to be very similar to tirzepatide,” she added.
Although the FDA’s ban on compounded semaglutide is based on the drug’s shortage, the agency has also stated safety concerns about the medications.
As of Feb. 28, the FDA said it had received more than 455 reports of adverse events related to people taking compounded semaglutide. These adverse events can be linked to dosing errors by patients or healthcare professionals, or to people taking doses beyond what’s in the FDA-approved drugs.
Though she hasn’t personally seen any patients who have had a bad outcome with compounded weight loss drugs, Kim said she does have concerns.
“Simply, compounded drugs are not FDA-approved, and safety is not guaranteed,” she said.
Though compounded drugs present a potential safety hazard, Carter-Johnson said she worries a ban on compounded semaglutide could actually make the situation less safe for patients, particularly if they try to circumvent the rule.
The FDA has already warned of counterfeit or illegal versions of Ozempic and other weight-loss drugs, some made overseas, being marketed in the U.S.
“Practically, these patients will turn to shady compounding pharmacies who will defy the FDA, putting themselves more at risk, as more trustworthy pharmacies comply with the FDA,” she said. “Others will turn to online or international versions of the drugs manufactured outside of FDA oversight and possibly counterfeit, with no active ingredients or dangerous contaminants.”
Because of this, Carter-Johnson and Kim agreed that weight loss drug patients should follow FDA guidance on medications. That means people currently taking compounded semaglutide should try to switch to Ozempic or Wegovy, or pursue a different method of weight loss.
However, this is easier said than done. Experts said those who can’t afford these brand-name weight loss medications will be the most affected by compounding bans.
“I wish patients had greater access to these medications, but we cannot guarantee the safety of compounded medications,” said Kim. “Both semaglutide and tirzepatide have an ongoing patent that does not expire until 2030. We need to work on more affordable options.”