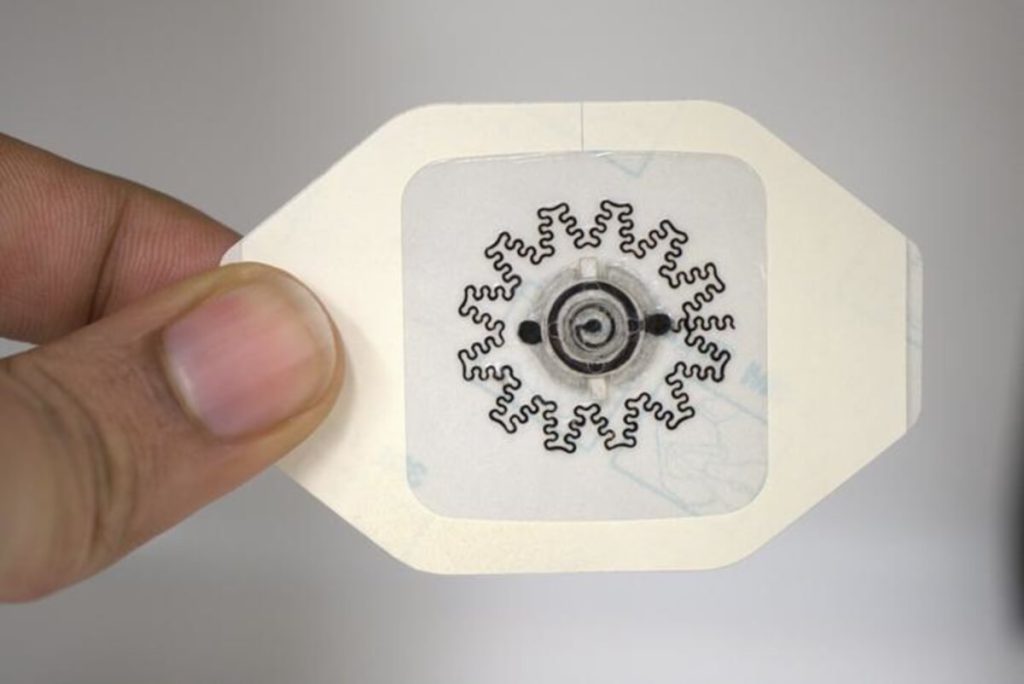
Photo of a water-powered, electronics-free dressing (WPED) for electrical stimulation of wounds. (Credit:
Rajaram Kaveti)
In a nutshell
- A new water-activated bandage generates healing electrical fields without any electronics, costing just $1 to produce compared to current treatments that can cost up to $20,000
- In testing with diabetic mice, 75% of wounds treated with the electrical bandage healed completely by day 11, versus 0% with standard bandages
- The technology allows patients to receive advanced wound treatment at home without visiting clinics or using bulky equipment, potentially improving treatment compliance
RALEIGH, N.C. — A drop of water might be all it takes to revolutionize wound healing. Researchers have developed a $1 bandage that, when activated with water, generates its own electrical field to speed healing. This could transform treatment for the millions of Americans who struggle with chronic wounds.
About 2% of Americans suffer from chronic wounds, which are injuries that stubbornly refuse to heal normally. These persistent wounds, like diabetic foot ulcers, often recur after treatment and significantly increase the risk of amputation and death. Current treatments range from basic bandages to advanced biological therapies, but they’re either minimally effective or prohibitively expensive, with some treatments costing upwards of $20,000 per wound.
The study, published in Science Advances, reveals a treatment alternative that could make chronic wound care accessible and affordable. An American research team created a water-powered, electronics-free dressing (WPED) that costs approximately $1 to produce. The smart bandage uses a combination of magnesium and silver/silver chloride to generate a healing electrical field when activated with water.
“Our goal here was to develop a far less expensive technology that accelerates healing in patients with chronic wounds,” says co-author Amay Bandodkar, assistant professor at North Carolina State University, in a statement. “We also wanted to make sure that the technology is easy enough for people to use at home, rather than something that patients can only receive in clinical settings.”


Rajaram Kaveti)
The dressing’s secret lies in its unique battery design. When water is added to a special inlet pad, it activates a flexible, biocompatible battery. This creates a gentle electrical field that helps stimulate the wound bed. A built-in “check pad” changes color to show when the separator is fully hydrated and the battery is working. The entire system weighs just 290 milligrams, only about 20% more than a standard bandage. Using specially designed electrodes, the bandage can conform to irregular wound surfaces.
“This ability to conform is critical because we want the electric field to be directed from the periphery of the wound toward the wound’s center,” says co-first author Rajaram Kaveti from North Carolina State University. “In order to focus the electric field effectively, you want electrodes to be in contact with the patient at both the periphery and center of the wound itself.”
Unlike existing electrical wound therapies that require bulky external equipment, this dressing works independently. After activation with water, it provides electrical stimulation for varying periods from 30 minutes to over 7 hours, depending on factors like wound characteristics and environmental conditions.
Laboratory testing revealed impressive resilience across temperatures and pressures. The dressing maintained function across a wide temperature range, though higher temperatures reduced duration due to faster water evaporation. It continued working under pressures similar to those experienced by heel wounds during walking.


Gurudatt Nanjanagudu Ganesh)
In carefully controlled trials with diabetic mice, the results showed significant promise. By day 11, 75% of wounds treated with the electrical dressing had fully closed, compared to 12.5% in the non-powered group and none in the control group. By day 13, healing rates reached 88% for the electrical dressing group.
“We found that the electrical stimulation from the device sped up the rate of wound closure, promoted new blood vessel formation, and reduced inflammation, all of which point to overall improved wound healing,” says co-first author Maggie Jakus, a graduate student at Columbia University.
“Diabetic foot ulceration is a serious problem that can lead to lower extremity amputations,” says co-author Aristidis Veves, professor of surgery at Beth Israel Deaconess Center. “There is urgent need for new therapeutic approaches, as the last one that was approved by the Food and Drug Administration was developed more than 25 years ago.”
While the researchers continue refining the technology for eventual clinical trials, their work highlights an important principle in medical innovation: sometimes the most impactful solutions aren’t the most complicated ones. By harnessing simple chemistry to replicate the body’s natural healing mechanisms, they’ve created a technology that could help millions access better wound care without breaking the bank.
Paper Summary
Methodology
The researchers built their dressing in several carefully designed layers. At its core is a ring-shaped battery designed to let doctors see through to the wound. The battery uses magnesium for its negative terminal and silver/silver chloride for its positive terminal, separated by a special cellulose membrane containing sodium chloride (table salt). When water reaches this membrane through an inlet pad, it activates the battery, creating an electrical field. The dressing includes a visual “check pad” that changes from light pink to blue to confirm proper activation, and returns to blue when the separator dries out. This setup avoids the need for any external power sources or complex electronics.
Results
The testing showed several key findings:
- The dressing provided electrical stimulation for varying periods (0.5 to >7 hours) depending on wound conditions
- It maintained function at temperatures from -20°C to 45°C, though duration decreased at higher temperatures
- In animal trials with 23 diabetic mice (8 electrical dressing, 8 non-powered dressing, 7 standard bandage):
- 75% of electrically treated wounds closed by day 11
- The electrically treated wounds showed 43% thicker new skin formation
- Blood vessel formation increased significantly compared to control groups
- Inflammation markers showed better healing patterns in the electrically treated group
Limitations
Several important limitations should be noted:
- The study primarily used mouse models, which don’t fully replicate human wound healing
- The current design requires daily replacement to maintain electrical stimulation
- Performance varies with environmental conditions like temperature and humidity
- The dressing’s effectiveness might vary with different wound types and locations
- Long-term safety and effectiveness in humans still needs to be evaluated through clinical trials
Takeaways and Discussion
The research demonstrates several significant advances:
- Proves that effective wound treatment can be achieved without complex electronics
- Matches healing rates of more expensive treatments at a fraction of the cost
- Functions without external power sources or complicated equipment
- Shows promise for treating diabetic wounds, which affect millions globally
- Could potentially make advanced wound care more accessible in resource-limited settings
Funding and Disclosures
The research received support from:
- Defense Advanced Research Projects Agency (grant D20AC00004)
- North Carolina State University Start-up Grant
- National Science Foundation (EEC-1160483)
- Joint Department of Biomedical Engineering’s REU program
Two authors (Kaveti and Bandodkar) filed a patent application related to this technology through North Carolina State University (D2024-0114, filed November 30, 2023).
Publication Information
The study was published in Science Advances (Volume 10, Article eado7538) on August 7, 2024. It is titled, “Water-powered, electronics-free dressings that electrically stimulate wounds for rapid wound closure.”