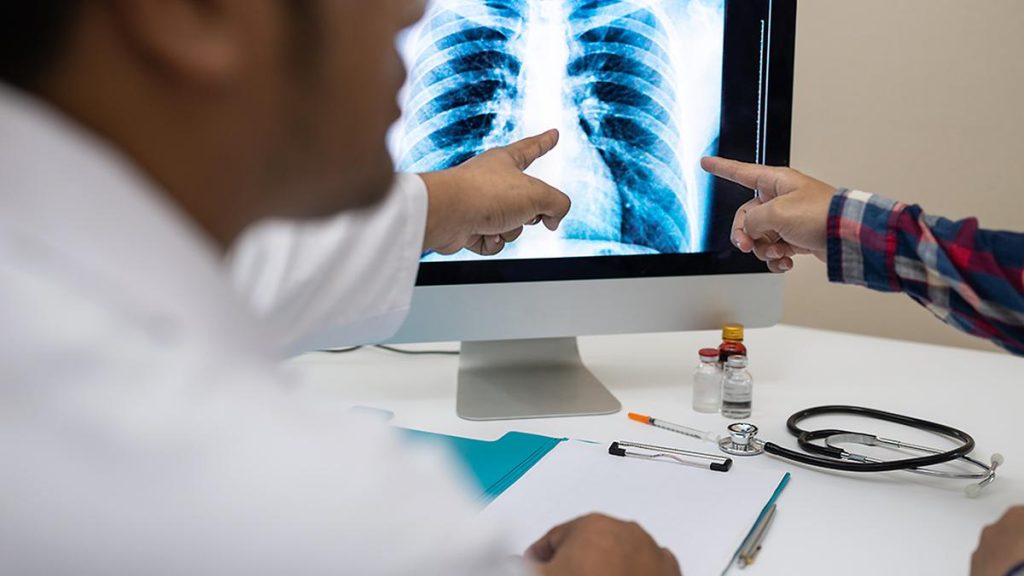
Even as time is running out to meet Prime Minister Narendra Modi’s goal of “eliminating” TB by 2025, the Health Ministry appears to be moving at glacial speed and is largely disinterested. A report submitted by the Health Technology Assessment of India (HTAIn) committee in February 2024 on two indigenously developed solutions to screen people with presumptive and subclinical TB using AI-assisted chest X-ray interpretation was posted on the HTAIn website only on December 6, 2024. The two indigenously developed solutions are qXR from the Bengaluru-based Qure.ai and Genki from Pune’s DeepTek.
HTA assessment of a new technology or tool for cost-effectiveness and efficacy in terms of sensitivity and specificity is not mandatory. However, the Central TB Division (CTD) waits for the HTA report and approval by the Medical Technology Assessment Board (MTAB) chaired by Niti Aayog and involves stakeholders including itself before programmatic implementation of any new tool, technology or treatment regimen using novel drugs for TB. For instance, TrueNat for TB diagnosis was validated by ICMR’s National Institute of Research in Tuberculosis (NIRT), Chennai before it was assessed by the HTA before it was included in the TB programme. Similarly, the BPaLM/BPaL regimen for MDR-TB treatment was tested in a phase 3/4 trial by NIRT and then assessed by HTA before being included in the TB programme by the Central TB Division.
Surprisingly, despite the assessment and approval of the two AI tools by the HTA and MTAB, respectively, the Central TB Division has not included them for programmatic implementation. However, even in the absence of an HTA assessment, the CTD has “recommended for programmatic introduction” a similar AI tool — DeepCXR — developed by the Institute for Plasma Research, Gandhinagar. An ICMR expert committee had approved the AI tool for “use under the national TB programme”.
Worse, CTD failed to officially communicate to the States that it was recommending the use of the DeepCXR tool in the TB programme. Instead, CTD informed the States in end-January this year to “consider utilising” the DeepCXR tool only when the States reached out to CTD seeking AI solutions to analyse chest X-ray images. And the 100-day TB elimination campaign began on December 7, 2024. As per CTD, the DeepCXR technology is available for free for use in the national TB programme.
As per a January-February 2020 review paper in the journal Neurology India, the Institute for Plasma Research had trained the AI tool using 6,000-30,000 chest X-ray images with an “overall accuracy of 93% on a test dataset”. Documents show that the AI tool has been trained using 54,000 X-rays and validated using 14,000 X-rays from “multiple datasets from more than 18 sites, with an average accuracy of over 96%”. There is no information on whether the DeepCXR tool is routinely used at any site for TB screening. Except for claims made in some documents and presentations, there is not a single published paper about the tool’s sensitivity and specificity and performance in field settings.
In contrast, there are a number of published studies on large patient populations where the performance of qXR and Genki has been evaluated. In fact, the qXR tool from Qure.ai with over 90% sensitivity and more than 70% specificity in people older than 15 years was one of the three AI algorithms that the WHO had referenced when updating the TB screening guidelines in March 2021. The qXR technology has been implemented in over 3,100 sites across 90 countries, and in about 490 sites in 25 States in India, and Genki has been implemented in over 80 sites across 15 States.
As per the HTA assessment, both qXR and Genki have been found to be cost-effective. The cost per case interpreted/screened is ₹30 in the case of qXR and ₹22 for Genki. The pooled sensitivity and specificity of the two interventions are 90% and 68%, respectively, which meets WHO’s non-inferior accuracy for TB screening. Both solutions were also found to be cost-effective. “Based on threshold analysis, the qXR technology will be cost-saving up to ₹400 per screening and Genki will be cost-effective up to ₹35 per screening,” says Dr. Somen Saha, Professor at the Indian Institute of Public Health (IIPH), Gandhinagar and the Principal Investigator of the HTA assessment committee for the two AI tools.
Useful interpretation
The importance of using chest X-rays for screening presumptive and subclinical TB cases cannot be overemphasised. 42.6% and 39% TB cases during the National TB Prevalence Survey 2019-2021 and TB prevalence survey in Tamil Nadu (2021-2022), respectively, were detected only because a chest X-ray was used for screening. AI-assisted X-ray interpretation takes less than a minute, has high accuracy, reduces the cost of TB detection, and can be used in resource-limited settings.
Published – February 22, 2025 09:20 pm IST